MammoMARK® & CorMARK®
BIOPSY SITE IDENTIFIERS
Rapid expansion anchors the marker in place for unsurpassed placement accuracy.1
MammoMARK® & CorMARK® Biopsy Site Identifiers
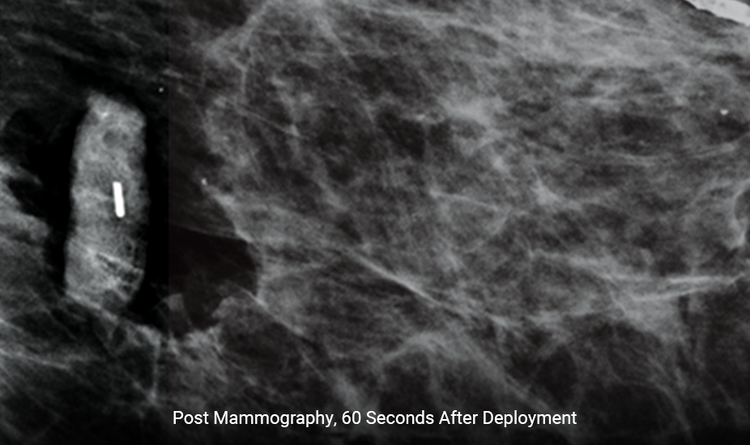
Expands Rapidly
The size increases by 300% within 60 seconds, reducing the likelihood of movement when releasing breast compression.1
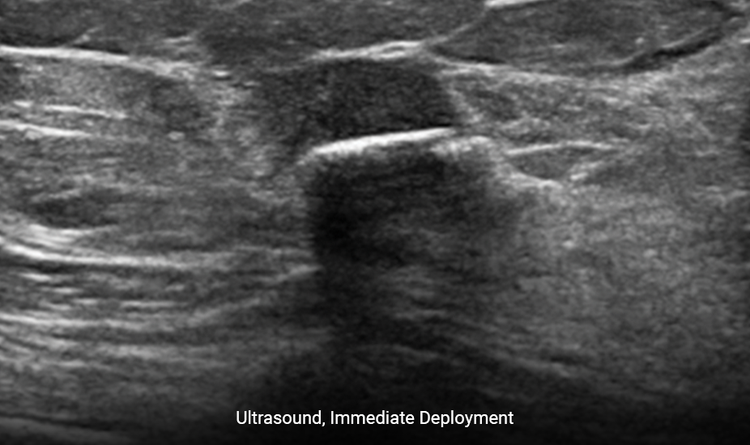
Provides Clear Ultrasound Visibility
Increased surface area provides ease of ultrasound visibility at time of deployment.2
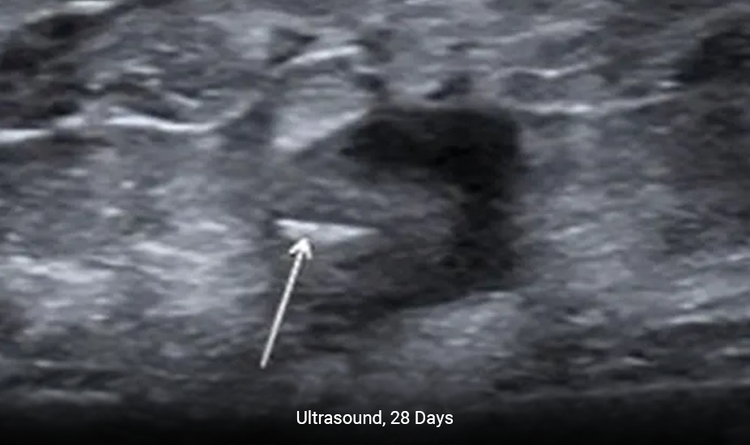
Enables Precise Placement
The MammoMARK® & CorMARK® markers help with accurate placement in the biopsy cavity.2

Absorbs Fluid Quickly
The MammoMARK® & CorMARK® markers assist in fluid management by quickly absorbing surrounding fluid.1
Three distinct shapes for better tracking of multiple biopsy sites
SHAPES
- Bowtie
- Triple Twist
- U-Shape



“The most significant advantage we found with the MammoMARK® is the
ability to consistently localize [it] using sonography.”
Krakos et al. Advantages of Using the New MammoMark Percutaneous Breast Biopsy Marker – a Large Center Experience.
FREQUENTLY ASKED QUESTIONS
What is the difference between the MammoMARK® & CorMARK® markers?
The applicators are the only difference between the two markers. The CorMARK® markers are the direct puncture applicators and the MammoMARK® markers encompass all others.
What are the MammoMARK® & CorMARK® markers composed of?
The MammoMARK® & CorMARK® markers are composed of bovine collagen and titanium.
Do the MammoMARK® & CorMARK® markers contain any nickel?
No, the markers do not contain any nickel.
RELATED PRODUCTS

HydroMARK™ Breast Biopsy Site Marker
Enduring exclusive hydrogel technology provides long-term ultrasound visibility in percutaneous breast biopsy procedures, including axillary lymph nodes.3

HydroMARK™ Plus Breast Biopsy Site Marker
Same great benefits of the original HydroMARK™ markers, with added features designed to alleviate displacement, enhance visibility and ease of locating.4,5,6

MammoSTAR® Biopsy Site Marker
The all-natural biopsy marker provides a non-metal marking alternative with a long-lasting beta glucan carrier for unique patient sensitivities.

LumiMARK™ Biopsy Site Marker
Three multifaceted nitinol shapes designed for easy identification from any angle.7

BiomarC® Biopsy Site Marker
All-natural design in a small size, ideal for superficial lesions.
1. MammoMARK® Device Test – PRC043442 Rev B, pgs. 2, 5-6: Collagen Expansion
2. Corsi F, Sorrentino L, Bossi D, Sartani A, Foschi D. Preoperative localization and surgical margins in conservative breast surgery. Int J Surg Oncol. 2013;2013:793819. doi:10.1155/2013/793819
3. Indication for lymph node using HydroMARK™ markers is limited to the United States with other country registrations pending.
4. HydroMARK™ Device Test – PCR-000414, Summative Usability
5. HydroMARK™ Design Plan – ADD-00013 Rev G, Page 15
6. HydroMARK™ Device Test – PCR-000299, SDR0098 Vacuum Suction Testing
7. LumiMARK™ Device Tests: PCR-000340 System Design, PCR-000579 Summative Usability, ES-002647 Claims Assessment
Products may not be approved or available in your region. Please check with your local Mammotome representative.