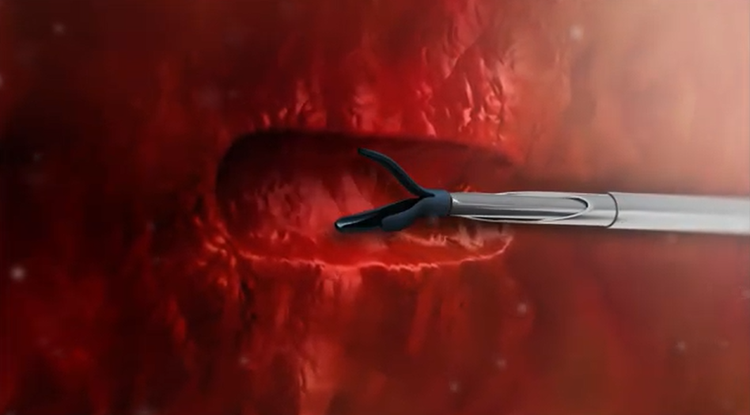
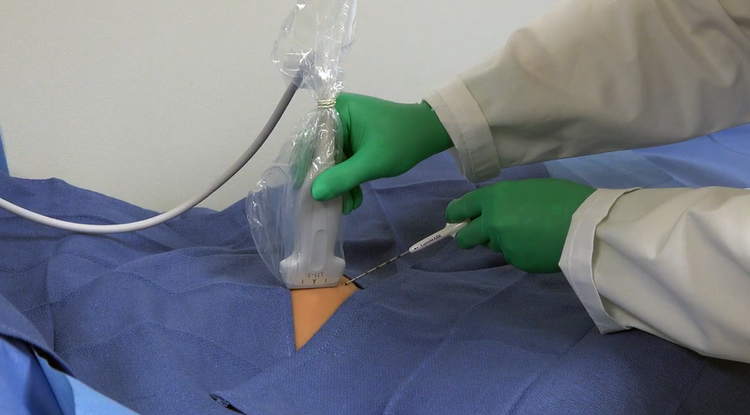
LumiMARK™
BIOPSY SITE MARKER
Three multifaceted nitinol shapes designed for easy identification from any angle.1
3D Shapes Distinct From Every Angle
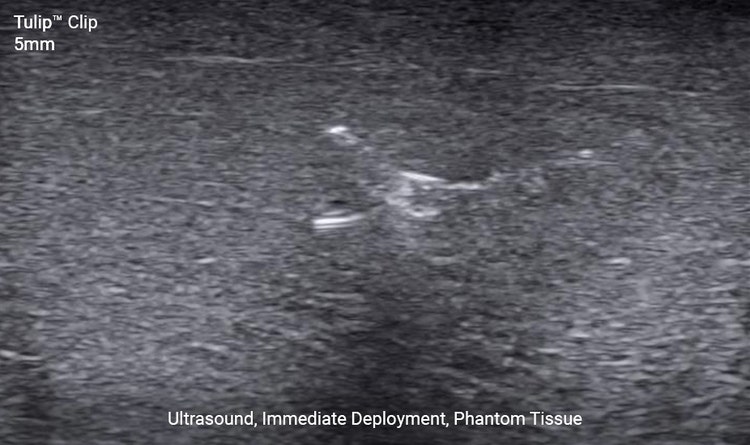
Ultrasound Visibility
These self-expanding markers deliver great ultrasound visibility by appearing hyperechoic both immediately and permanently.1

Options with Purpose
The 5mm TulipTM clip may be selected for use cases needing a smaller marker. The 10mm RoseTM clip is designed for effortless locating under ultrasound.2
Three Biopsy Site Markers
- TulipTM clip
- LotusTM clip
- RoseTM clip



Learn More About LumiMARK™ Markers
Would you like to know more about the LumiMARK™ markers?
FREQUENTLY ASKED QUESTIONS
What are the LumiMARK™ markers composed of?
The LumiMARK™ markers are composed of nitinol with no resorbing carrier. The nitinol (grade S) metal is comprised of nickel, titanium, and other minor constituents.
Do the LumiMARK™ markers contain nickel?
The LumiMARK™ markers do contain nickel. Do not use on patients with known nickel allergies.
Why are the LumiMARK™ markers made of nitinol?
Nitinol is a metal alloy that offers shape memory allowing the distinctive markers to be compressed and take full shape upon deployment.
What size are the LumiMARK™ markers?
The TulipTM clip is 5mm (.20”) x 5mm (.20”).
The LotusTM clip is 7mm (.27”) x 4mm (.16”).
The RoseTM clip is 10mm (.39”) x 5mm (.20”).
Can you use the LumiMARK™ markers in lymph nodes?
The LumiMARK™ Biopsy Site Marker is indicated to mark tissue associated with a percutaneous breast biopsy procedure, including axillary lymph nodes, and be permanently visible under ultrasound, X-ray and MRI.
Are the LumiMARK™ markers MR safe?
The applicator with metal cannula is not safe for the MR environment. These markers are MR conditional, meaning a patient can be safely scanned in an MRI system meeting the criteria set in the IFU.
RELATED PRODUCTS

HydroMARK™ Breast Biopsy Site Marker
Enduring exclusive hydrogel technology provides long-term ultrasound visibility in percutaneous breast biopsy procedures, including axillary lymph nodes.3

HydroMARK™ Plus Breast Biopsy Site Marker
Same great benefits of the original HydroMARK™ markers, with added features designed to alleviate displacement, enhance visibility and ease of locating.4,5,6

MammoMARK® & CorMARK® Biopsy Site Identifier
Rapid collagen expansion anchors the marker within the biopsy cavity reducing the likelihood of movement.7,8

MammoSTAR® Biopsy Site Marker
The all-natural biopsy marker provides a non-metal marking alternative with a long-lasting beta glucan carrier for unique patient sensitivities.

BiomarC® Biopsy Site Marker
All-natural design in a small size, ideal for superficial lesions.
1. LumiMARK™ Device Tests: PCR-000340 System Design, PCR-000579 Summative Usability, ES-002647 Claims Assessment
2. LumiMARK™ Device Tests: PCR-000579 Summative Usability and ES-002647 Claims Assessment
3. Indication for lymph node using HydroMARKTM markers is limited to the United States with other country registrations pending.
4. HydroMARKTM Device Test – PCR-000414, Summative Usability
5. HydroMARKTM Design Plan – ADD-00013 Rev G, Page 15
6. HydroMARKTM Device Test – PCR-000299, SDR0098 Vacuum Suction Testing
7. MammoMARK® Device Test – PRC043442 Rev B, pgs. 2, 5-6: Collagen Expansion
8. Corsi, F., Sorrentino, L., Sartani, A., Bossi, D., Amadori, R., Nebuloni, M., Truffi, M., Bonzini, M., & Foschi, D. (2015). Localization of nonpalpable breast lesions with sonographically visible clip: Optimizing tailored resection and clear margins. The American Journal of Surgery, 209(6), 950-958
Products may not be approved or available in your region. Please check with your local Mammotome representative.