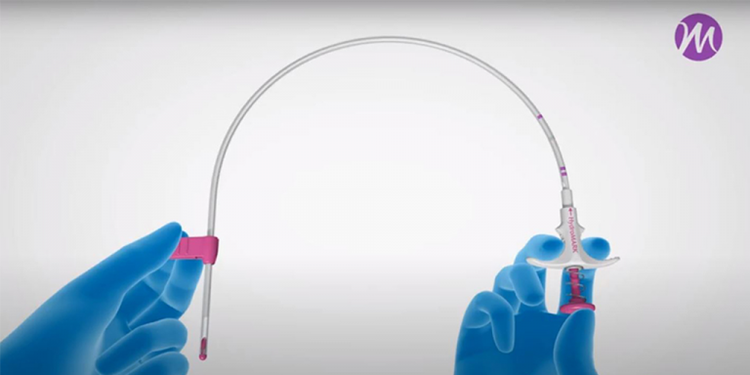
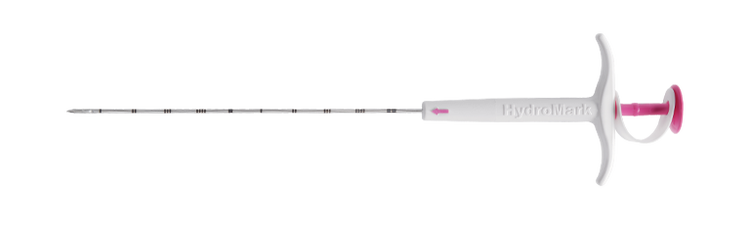
HydroMARK™
BREAST BIOPSY SITE MARKERS
Exclusive hydrogel technology provides clinicians with long-term ultrasound visibility.1,2
HydroMARK™ Breast Biopsy Site Markers
Highly Visible
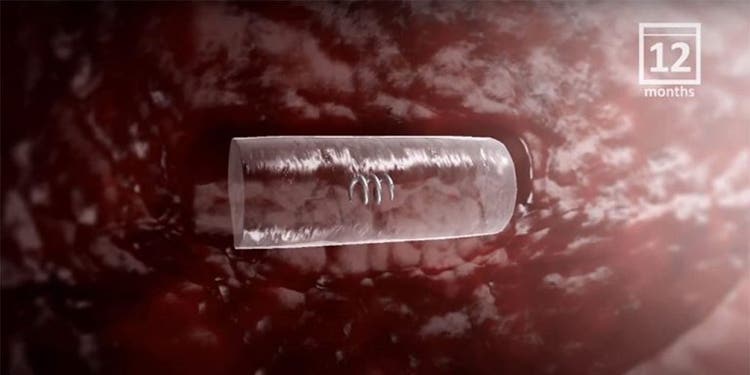
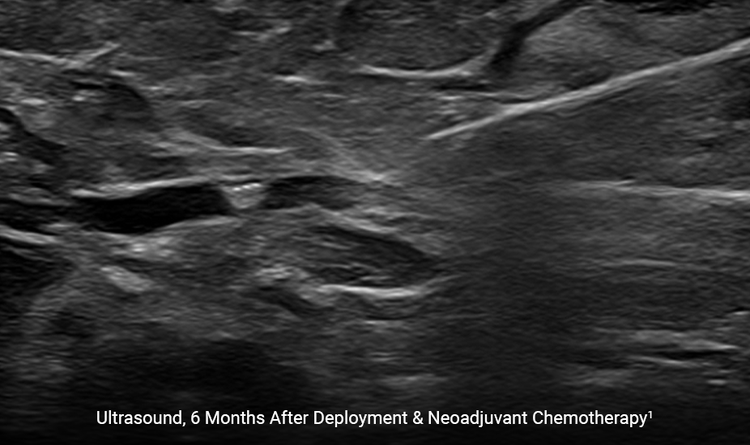
The HydroMARK™ markers hydrate to provide long-term visibility even after neoadjuvant chemotherapy.2
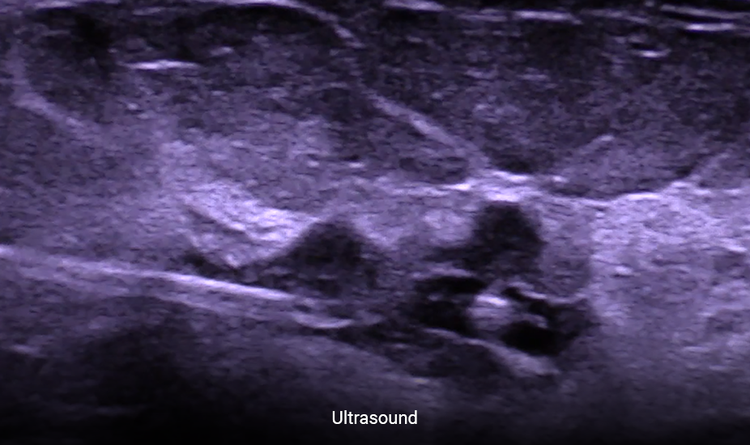
Allows Easier Localization
Consistently place any localization device under ultrasound.3
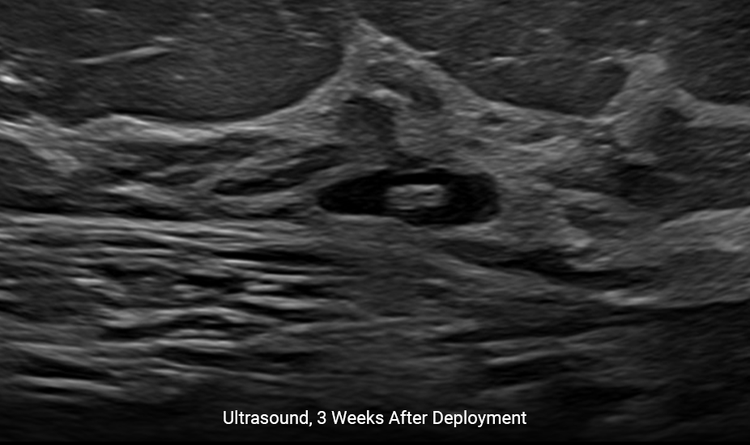
Creates a Better Patient Experience
Localization under ultrasound provides a faster, more comfortable procedure for the patient.4
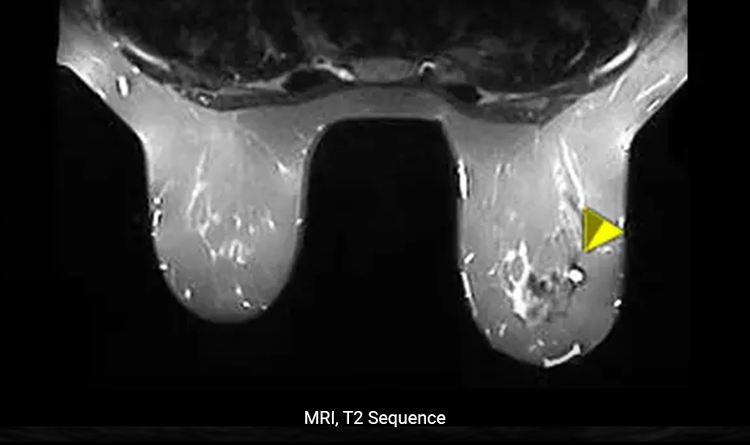
Conspicuity Under MRI
The HydroMARK™ markers are uniquely distinguishable during a T2 sequence.5
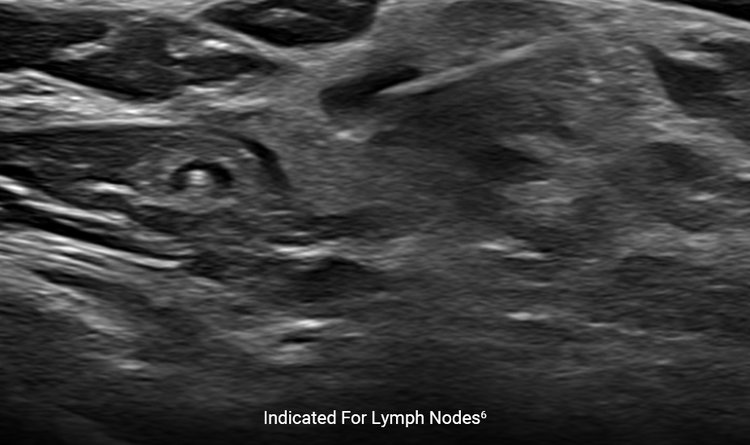
Lymph Node Indication
The HydroMARK™ direct puncture applicators are indicated to mark tissue during a percutaneous breast biopsy procedure, including axillary lymph nodes.6
Three distinct shapes for visibility
- Open Coil
- Butterfly
- Barrel


“We use the HydroMARK™ marker exclusively for all of our biopsies. We have had such a great experience with the HydroMARK™ marker that we need no other clip.”
Robin B. Shermis, MD, MPH, FACR
Medical Director, ProMedica Breast Care on the campus of The Toledo Hospital
FREQUENTLY ASKED QUESTIONS
What is the composition of the hydrogel?
The hydrogel is composed of polyethylene glycol (PEG).
Does the HydroMARK™ marker contain nickel?
The HydroMARK™ stainless steel marker products contain a percentage of nickel. The HydroMARK™ titanium products contain no nickel.
What is the duration of the HydroMARK™ marker’s enhanced long-term visibility?
Studies have shown that visibility can last up to 12 months in some patients.1
Can you use HydroMARK™ markers in lymph nodes?
The HydroMARK™ direct puncture applicators are indicated to mark tissue during a percutaneous breast biopsy procedure, including axillary lymph nodes.6
RELATED PRODUCTS

HydroMARK™ Plus Breast Biopsy Site Marker
Same great benefits of the original HydroMARK™ markers, with added features designed to alleviate displacement, enhance visibility and ease of locating.7,8,9

MammoSTAR® Biopsy Site Marker
The all-natural biopsy marker provides a non-metal marking alternative with a long-lasting beta glucan carrier for unique patient sensitivities.

MammoMARK® & CorMARK® Biopsy Site Identifier
Rapid collagen expansion anchors the marker within the biopsy cavity reducing the likelihood of movement.10

LumiMARK™ Biopsy Site Marker
Three multifaceted nitinol shapes designed for easy identification from any angle.11

BiomarC® Biopsy Site Marker
All-natural design in a small size, ideal for superficial lesions.
1. Sakamoto, N., Fukuma, E., Tsunoda, Y. et al. Evaluation of the dislocation and long-term sonographic detectability of a hydrogel based breast biopsy site marker. Breast Cancer 25, 575–582 (2018). https://doi.org/10.1007/s12282-018-0854-8
2. Pinkney, Journal of Diagnostic Medical Sonography 29(4) 151 – 158, DOI 10.1177/8756479313486962
3. (Blumencranz, Ann. Surg. Oncol. (2014) 21:3273-3277, DOI 10.1245/s10434-014-3917-x)
4. (Gentile, Ann Surg Oncol. 2016 Oct;23(10):3284-9. doi: 10.1245/s10434-016-5325-x. Epub 2016 Jun 23.)
5. (Shah, Clin Imaging. 2019 May – Jun;55:196-212. doi: 10.1016/j.clinimag.2019.05.014. Epub 2019 Jun 14.)
6. Indication for lymph node using HydroMARK™ markers is limited to the United States with other country registrations pending.
7. HydroMARK™ Device Test – PCR-000414, Summative Usability
8. HydroMARK™ Design Plan – ADD-00013 Rev G, Page 15
9. HydroMARK™ Device Test – PCR-000299, SDR0098 Vacuum Suction Testing
10. MammoMARK® Device Test – PRC043442 Rev B, pgs. 2, 5-6: Collagen Expansion
11. LumiMARK™ Device Tests: PCR-000340 System Design, PCR-000579 Summative Usability, ES-002647 Claims Assessment
Products may not be approved or available in your region. Please check with your local Mammotome representative.