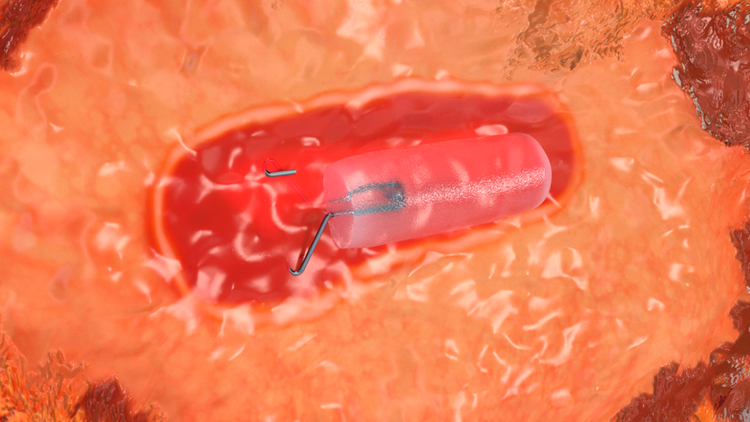
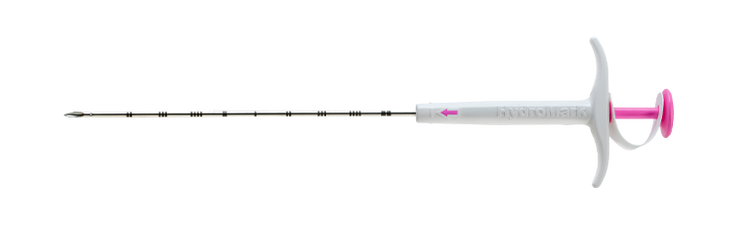
HydroMARK™ Plus
BREAST BIOPSY SITE MARKER
Same great benefits of the original HydroMARK™ markers, with added features designed to alleviate displacement, enhance visibility, and ease of locating.1, 2, 3
HydroMARK™ Plus Breast Biopsy Site Marker
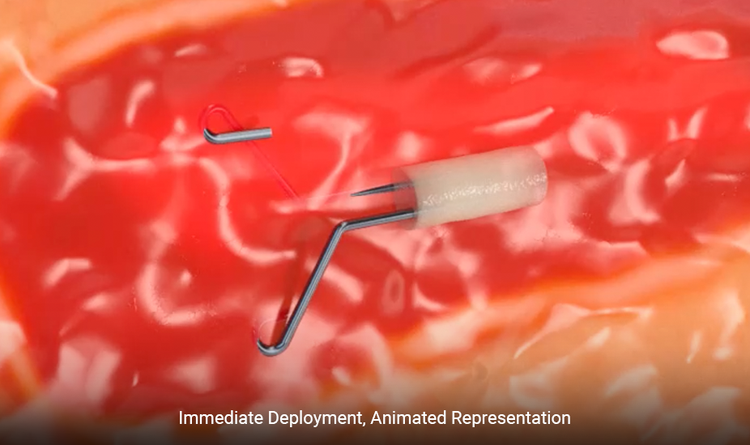
Attachment to Tissue
“Wings” are designed to anchor to the tissue to mitigate displacement during surgical excision.2
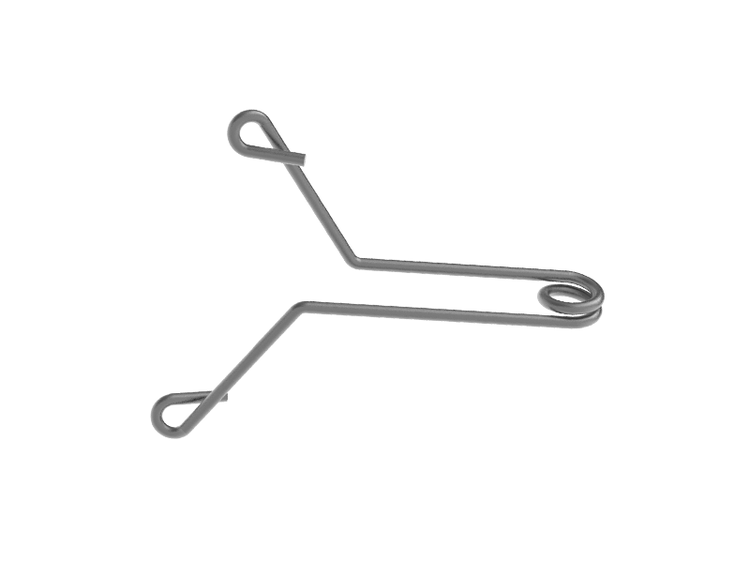
Enhanced Distinguishability
The Dragonfly™ clip is distinct and discernably man-made to breast anatomy.
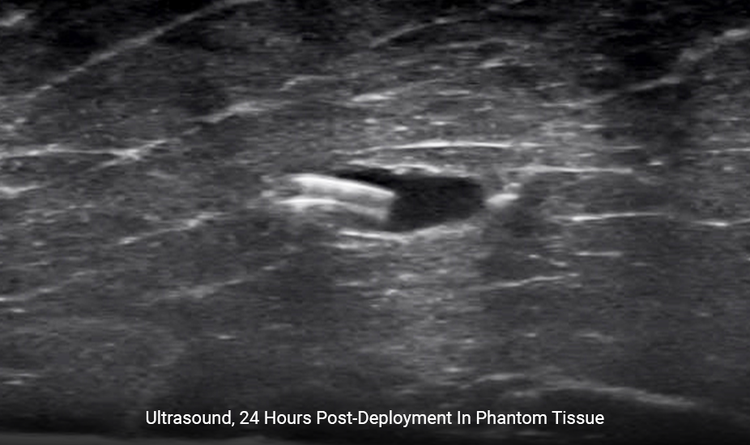
Ease of Locating
A portion of the clip is outside of the hydrogel offering additional echogenicity.
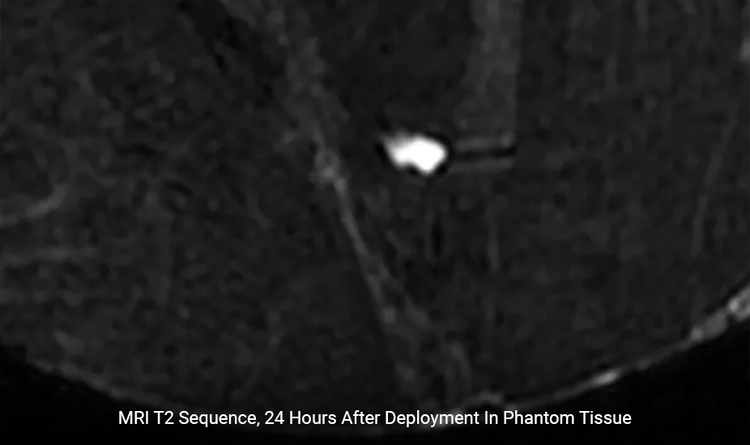
Minimal Artifact
Artifact is 1 x 1mm under Spin Echo and 3 x 3mm under Gradient Echo.4
Unique shape with "wings" designed to attach to breast tissue
- Dragonfly™ clip
“I am excited for this innovative biopsy marker to come to market. After seeing the tabletop test on the HydroMARK™ Plus Breast Biopsy Site Marker, I look forward to having a solution that addresses the need for a nickel-free, long-term, ultrasound visible clip that will not displace for the surgeons.”
Dr. Evita Singh
Director of Breast Imaging, Karmanos Cancer Institute
FREQUENTLY ASKED QUESTIONS
What is the composition of the hydrogel?
The hydrogel is composed of polyethylene glycol (PEG).
Does the HydroMARK™ Plus marker contain nickel?
The HydroMARK™ Plus titanium marker contains no nickel.
What is the duration of the HydroMARK™ Plus marker’s enhanced long-term visibility?
Studies have shown that visibility can last up to 12 months in some patients.5
Can you use the HydroMARK™ Plus markers in lymph nodes?
The HydroMARK™ direct puncture applicators are indicated to mark tissue during a percutaneous breast biopsy procedure, including axillary lymph nodes.6
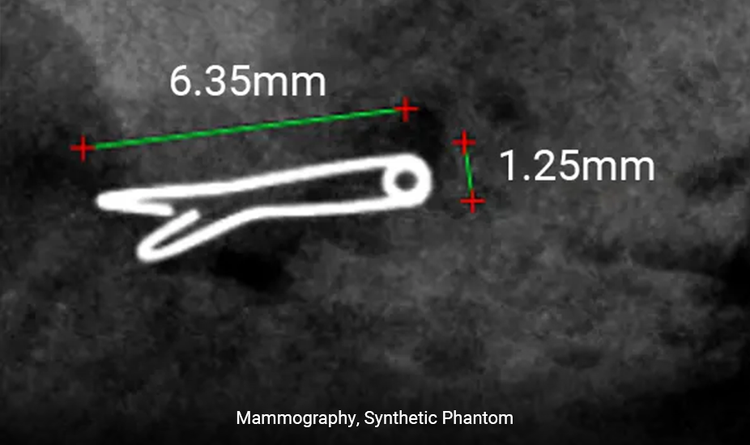
What size is the HydroMARK™ Plus Dragonfly™ clip?
The Dragonfly™ clip is 6.35mm (0.25”) x 5.08mm (0.2”) not including the hydrogel component.
How much of the marker extends outside of the hydrogel?
The bare portion of the marker outside of the hydrogel may vary in length depending on hydration, up to 3mm (0.12”).
RELATED PRODUCTS

HydroMARK™ Breast Biopsy Site Marker
Enduring exclusive hydrogel technology provides long-term ultrasound visibility in percutaneous breast biopsy procedures, including axillary lymph nodes.6

MammoSTAR® Biopsy Site Marker
The all-natural biopsy marker provides a non-metal marking alternative with a long-lasting beta glucan carrier for unique patient sensitivities.

MammoMARK® & CorMARK® Biopsy Site Identifier
Rapid collagen expansion anchors the marker within the biopsy cavity reducing the likelihood of movement.7

LumiMARK™ Biopsy Site Marker
Three multifaceted nitinol shapes designed for easy identification from any angle.8

BiomarC® Biopsy Site Marker
All-natural design in a small size, ideal for superficial lesions.
1. HydroMARKTM Device Test – PCR-000414, Summative Usability
2. HydroMARKTM Design Plan – ADD-00013 Rev G, Page 15
3. HydroMARKTM Device Test – PCR-000299, SDR0098 Vacuum Suction Testing
4. HydroMARKTM Device Test – PCR-000305
5. Sakamoto, N., Fukuma, E., Tsunoda, Y. et al. Evaluation of the dislocation and long-term sonographic detectability of a hydrogel-based breast biopsy site marker. Breast Cancer 25, 575–582 (2018). https://doi.org/10.1007/s12282-018-0854-8)
6. Indication for lymph node using HydroMARKTM markers is limited to the United States with other country registrations pending.
7. MammoMARK® Device Test – PRC043442 Rev B, pgs. 2, 5-6: Collagen Expansion
8. LumiMARK™ Device Tests: PCR-000340 System Design, PCR-000579 Summative Usability, ES-002647 Claims Assessment
Products may not be approved or available in your region. Please check with your local Mammotome representative.