MammoSTAR®
BIOPSY SITE MARKERS
Providing a non-metal marking alternative with a long-lasting
beta glucan carrier plug for unique patient sensitivities.
MammoSTAR® Biopsy Site Markers

Provides a Naturally Reassuring Option
The MammoSTAR® marker offers a non-metallic option composed of all natural minerals, Carbon Coated Zirconium Oxide and lyophilized beta-glucan gel (or simple sugar).
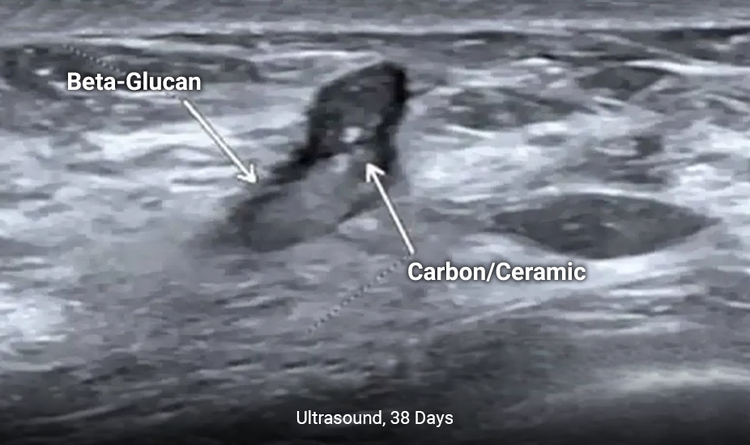
Enables Enhanced Long-Term Ultrasound Visibility
The MammoSTAR® marker provides enhanced long-term ultrasound visibility to allow easier pre-surgery localization.1
Two natural, non-metal shapes for better tracking of multiple biopsy sites
SHAPES
- Barbell (also available in a petite carrier)
- Tribell


FREQUENTLY ASKED QUESTIONS
Do MammoSTAR® markers provide enhanced ultrasound visibility?
Yes, the lyophilized beta-glucan gel (or simple sugar) provides enhanced long-term ultrasound visibility to allow easier pre-surgery localization.2
What is the MammoSTAR® marker composed of?
The MammoSTAR® marker is a pyrolytic carbon coating while the carrier is a lyophilized beta-glucan gel or simple sugar.
Does the MammoSTAR® marker contain nickel?
The MammoSTAR® marker does not contain nickel.
RELATED PRODUCTS

HydroMARK™ Breast Biopsy Site Marker
Enduring exclusive hydrogel technology provides long-term ultrasound visibility in percutaneous breast biopsy procedures, including axillary lymph nodes.3

HydroMARK™ Plus Breast Biopsy Site Marker
Same great benefits of the original HydroMARK™ markers, with added features designed to alleviate displacement, enhance visibility and ease of locating.4,5,6

MammoMARK® & CorMARK® Biopsy Site Identifier
Rapid collagen expansion anchors the marker within the biopsy cavity reducing the likelihood of movement.7

LumiMARK™ Biopsy Site Marker
Three multifaceted nitinol shapes designed for easy identification from any angle.8

BiomarC® Biopsy Site Marker
All-natural design in a small size, ideal for superficial lesions.
1. Mammotome Data: visibility and localization
2. Mammotome Data: visibility
3. Indication for lymph node using HydroMARK™ markers is limited to the United States with other country registrations pending.
4. HydroMARK™ Device Test – PCR-000414, Summative Usability
5. HydroMARK™ Design Plan – ADD-00013 Rev G, Page 15
6. HydroMARK™ Device Test – PCR-000299, SDR0098 Vacuum Suction Testing
7. MammoMARK® Device Test – PRC043442 Rev B, pgs. 2, 5-6: Collagen Expansion
8. LumiMARK™ Device Tests: PCR-000340 System Design, PCR-000579 Summative Usability, ES-002647 Claims Assessment
Products may not be approved or available in your region. Please check with your local Mammotome representative.
