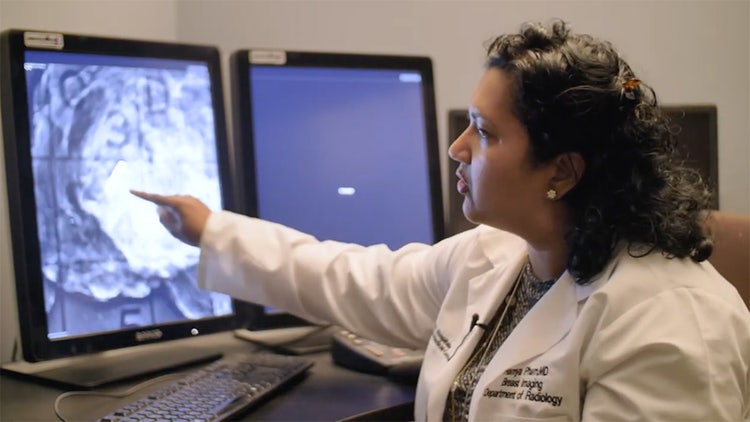
Magseed®
MAGNETIC SEED LOCALIZATION
A simple and effective alternative to wire localization. So far, it’s been used in over 250,000 localizations.1
The Marker Designed for Both Patients and Physicians

Enables Placement Flexibility
Forget ‘day of surgery’ constraints associated with wire-guided localization, now you can choose when and where to place the Magseed® marker, decoupling radiology and surgery schedules.

Ensures Reliable Implantation
Once in place, patients can simply forget about their Magseed® marker. It stays in place – whether it’s one day or a hundred days before surgery.2

Facilitates Confident and Consistent Retrieval
The Magseed® marker is easy to find, whether it’s a shallow lesion or more than 10 cm deep in the breast, resulting in a 99.9% retrieval rate across all 10,000 patients in clinical trials.2

Creates a Better Patient Experience
With the Magseed® marker, patients have been shown to feel less pain than using wires. They can return to normal daily life, reduce the number of visits to hospitals, decrease time spent in the hospital and eliminate delays to the operating room on the day of surgery. 3

Allows Better Cosmetic Outcomes
As the seed is minimally invasive, smaller incisions and a broad array of oncoplastic techniques become possible – giving your patients better outcomes than they may have thought possible.4
Tiny Seed. Huge Impact.
- The seed is 1x5mm, smaller than an uncooked grain of rice, and is inserted using a small 18-gauge needle.
- Highly visible on both ultrasound and X-ray, allowing you to comfortably place the seed, just like a biopsy clip.
- Can be implanted at any time before surgery, enabling you to separate radiology and surgery schedules.
- Unlike guidewires, the Magseed® marker’s special woven design means it will sit firmly implanted in the cancer and won’t move.
- Made of special material, the Magseed® marker is designed to never malfunction.
- Can be sensed from any angle, allowing full freedom in your surgical approach.


See How It Works
Would you like to know more about Magseed® wire-free lesion localization?
Why Magnetic Seeds?
Magnetic seeds have been designed to simplify breast cancer treatment, from the time the cancer is marked through to when it’s removed in surgery.

Accurate Placement
With the choice of when and where to mark the cancer, you have ultimate control over placement.

Firmly Implanted
Magnetic seeds won't move, break, or deactivate — no matter when they’re implanted.

Pinpoint Detection
Detectable to the millimeter, magnetic seeds guide you to the tumor with precision.

Accurate Removal
Being certain of the cancer’s location means you can remove it while leaving behind as much healthy tissue as possible.
FREQUENTLY ASKED QUESTIONS
Do I have to worry about the seed moving or breaking before surgery?
Unlike conventional guidewires, once a Magseed® marker has been placed, patients won’t be able to feel it and its special design means it will sit firmly implanted in the cancer. From the time it’s placed to the time of the surgery, it can’t be dislodged or broken.
Is the Magseed® marker composed of tiny magnets?
No, the Magseed® marker is made of medical grade stainless steel (low nickel) that becomes temporarily magnetized when the Sentimag® probe is near. This facilitates the ability to perform precision localization procedures.
Why is the Magseed® marker a better solution than traditional wire localization for breast cancer surgery?
The Magseed® marker can be placed days, weeks or months ahead of surgery to suit both you and your patients’ schedules. The procedure is simple and can often be completed in a matter of minutes. This flexibility means you don’t have to worry about additional procedures on the day of surgery affecting scheduling.
RELATED PRODUCTS

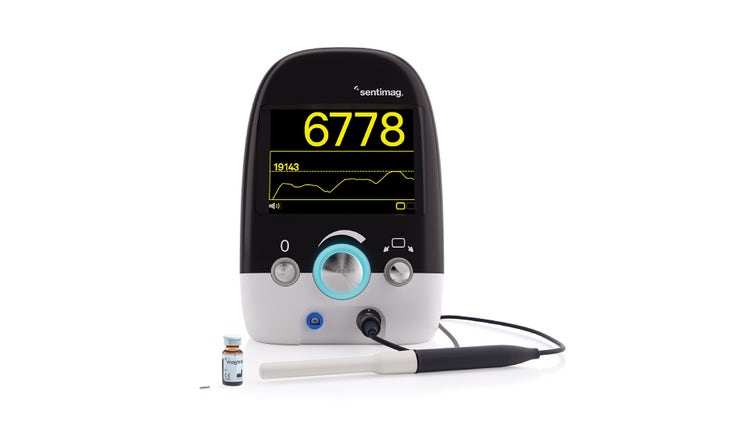
Sentimag® Localization Platform
Four techniques in one platform allow for more surgical options than any other non-gamma localization system.
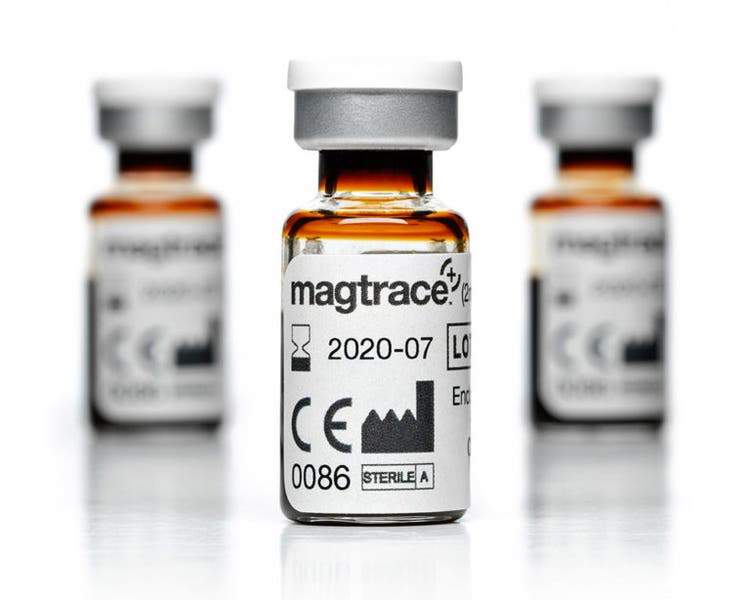
Magtrace® Lymphatic Tracer
The Magtrace® Lymphatic Tracer is engineered to be uniform and small enough for rapid migration, yet large enough to be mechanically filtered by the sentinel lymph nodes.

HydroMARK™ Breast Biopsy Marker
The HydroMARK™ marker’s exclusive hydrogel technology provides clinicians with unmatched long-term ultrasound visibility.
XPERT® 40 Specimen Radiography System
When you need to identify fine details, in a wide range of specimens, in a short amount of time.
1. Data on file at Endomag
2. Gera, R. (2020) Evolving Role of Magseed in Wireless Localization of Breast Lesions: Systematic Review and Pooled Analysis of 1,559 Procedures. Anticancer Research
3. Kühn F, Simon CEE, Aliyeva I, KUßMAUL J, GROß J, Schweizerhof O, Blohmer JU, Karsten MM. A German Study Comparing Standard Wire Localization With Magnetic Seed Localization of Non-palpable Breast Lesions. In Vivo. 2020 May-Jun;34(3):1159-1164. doi: 10.21873/invivo.11888. PMID: 32354905; PMCID: PMC7279781.
4. Pieszko et al (2020) Evaluation of the Nonradioactive Inducible Magnetic Seed System Magseed for Preoperative Localization of Nonpalpable Breast Lesions – Initial Clinical Experience.